
Research & Development (R&D)
Research & Development
Research &
Development
- Top
- Research & Development (R&D)
Based on the idea that "technologies that enhances quality of life should not be influenced by transient fads and that what is truly useful will inevitably become standard.", we are conducting research and development with various partners, aiming at ideas that could become the standards of the future.
The values and strengths we promise
Value
-
We will pursue and provide product services that emphasize the "optimal" fit for each individual, rather than aiming for the maximum, by making the most of D2C's direct connection with customers. (Optimal rather than maximum®)
-
We will continue to create richer, more enjoyable and more efficient options by leveraging our strengths in direct marketing, creativity and hospitality.
-
We don't chase trends. We develop and implement ideas and technologies that enhances quality of life that could become future standards.
-
We pursue reliable quality based on world patented technology and evidence. We do not produce anything without proof, based on world-class advanced technology.
-
We offer products and services that are not driven by market demand, but rather by the passion and dedication of our employees. These products are created with a genuine desire to provide something of value to those who matter most to us.
-
As manufacturing and sales increase, so too will the natural environment that is the source of this. This will create a virtuous cycle in which the environment becomes richer as a result of our activities. (Sustainable Cycle Supplement® Concept)
Initiatives taken
Initiative
One of our development ingredients is "Vita-Brid C". Our hybrid powder combines all-purpose vitamin C*1 and essential minerals in a unique structure and has achieved penetration※2 of pure vitamin C*1 for about 12 hours using special technology recognized around the world. This technology was developed by a team of leading researchers, including Nobel Prize judges, for the development of anti-cancer drugs.
We use a drug delivery system (DDS) to combine these ingredients in a way that allows the vitamin C※1 to be absorbed by the body for a longer period of time.
By incorporating pure vitamin C*1 ingredients between layers of LDH*1 and allowing for expansion and contraction, we have developed a method of gradual and stable ingredient distribution. Once applied to the skin, the product binds with waste products such as sweat (NaCl) and other unwanted substances that arise from natural skin metabolism. In exchange, the vitamin C*1 inside is released and exchanged (ion exchange method), enabling deep penetration into the skin*1 for an extended period.

It would be a great privilege to be able to make "Vita-Brid C" available to people all over the world.
developer of Vita-Brid C
2008 Fellow of the Royal Society of Chemistry
2007 Korea's Highest Science and Technology Award
2004 French Government Order of Merit, etc.
Ph.D. in Materials Engineering, Tokyo Institute of Technology Graduate School
Ph.D. in Chemistry, University of Munich Graduate School, etc.
The key to the development of "Vita-Brid C" was LDH※3, a layered double hydroxide. Initially, this nanomaterial was used as a drug delivery system.
It is well known that vitamin C※1 is a wonderful ingredient, but at the time there were no precedents in the world for cosmetics that used a technology to stabilize vitamin C※1 as it is. This is how Professor Jin-ho Choy, the developer of VITABRID C, came up with the idea that "using LDH※3 as a technology to stabilize vitamin C※1 might be a good idea".

LDH※3 was published in the renowned German chemistry journal "Angewandte Chemie" and was also featured in the highlights of the academic journal "Nature". Furthermore, it was selected as one of the world's eight most promising technologies in the American Chemical Society journal. We initiated research to commercialize the technology with the aspiration of making it a valuable resource for everyone.

LDH※3 is a mineral※3 (zinc) that is essential for human life. As a new substance that fuses (i.e., hybridizes) a beneficial mineral with vitamin C*1, Professor Jin-ho Choy named it "Vita-Brid C" to reflect its unique combination of mineral*3 and vitamin C*1.
The creation and application of hybrids represents the fruit of Professor Jin-ho Choy's lifelong research, which has spanned approximately 30 to 40 years.

The Vita-Brid C, developed subsequently, also garnered attention at Cosmoprof, one of the largest international beauty exhibitions in Asia. Approximately 670 companies from around the world visited the exhibition. In 2016, it won the Trend Setter Award for setting the tone for the next era.
Furthermore, Vita-Brid C has received considerable recognition in Japan and abroad, including the "Grand Gold Quality Award" for two consecutive years (2016 and 2017) at the Monde Selection, where the quality and value of lifestyle products are evaluated by experts.
*2 Reaches the stratum corneum
*3 Astringent ingredients
Original ingredient “Peptibrid ®”.
Peptides are renowned for their ability to provide the skin with a firm and elastic feel, and are increasingly sought after as a moisturizing ingredient. However, one of the challenges associated with peptides is their difficulty in penetrating the skin.
After conducting extensive research, we developed organic-inorganic hybrid technologies based on LDH※3 to address this challenge. Our solution improves peptide absorption by dispersing peptides, which tend to clump, and transmitting them to the skin in a stable form through zinc oxide, a bio-compatible mineral※3. The peptides are then stored in LDH※3 layers.
We have developed a unique ingredient called “Peptibrid®” that applies this DDS (Drug Delivery System) technology to stabilize peptides and enhance their penetration into the skin.
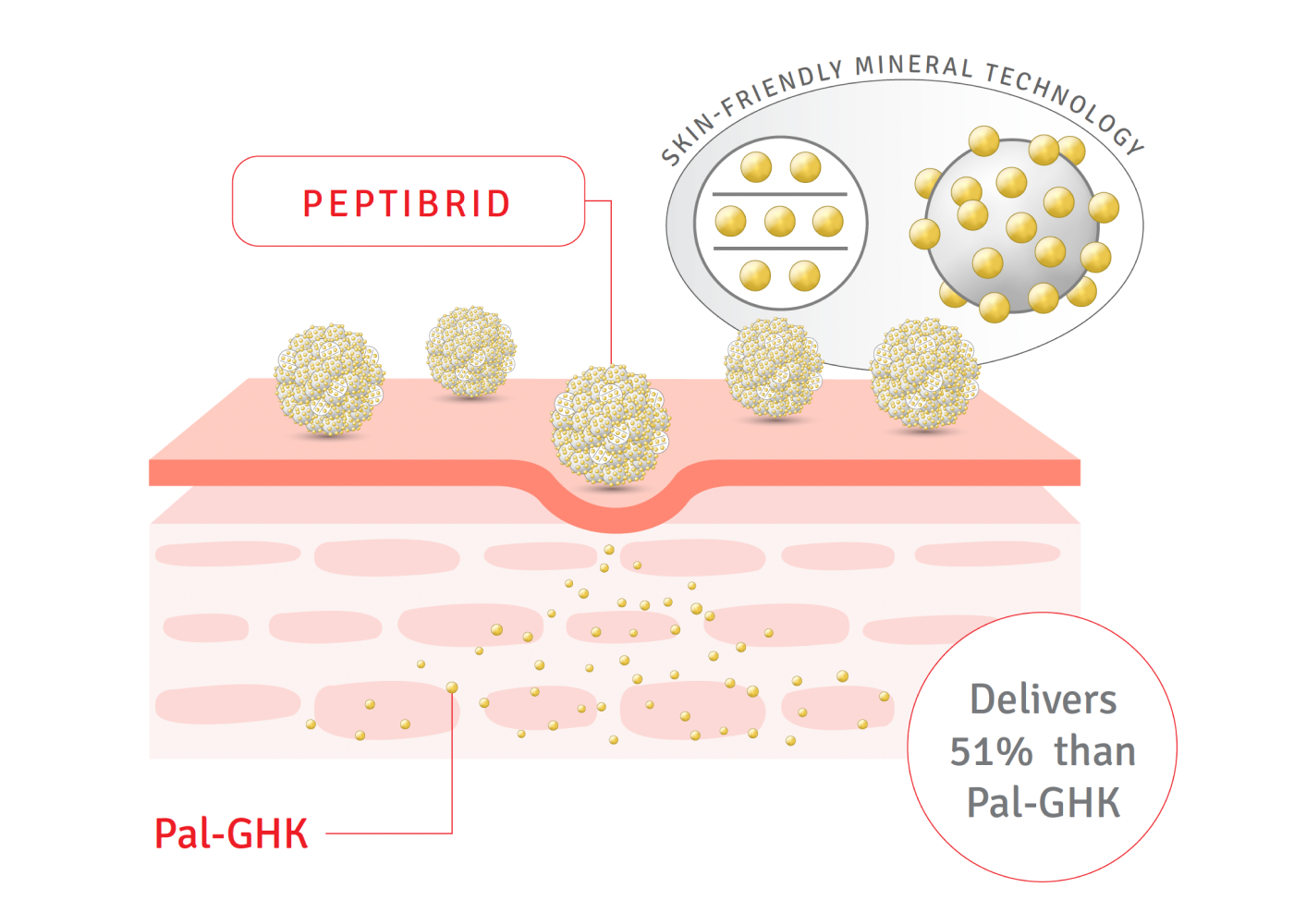
It also incorporates the hybrid technology of “Vitabrid C” and “Peptibrid®”.
While "Vita-Brid C" provides long-lasting penetration to support radiant skin, the unique ingredient "PeptiBrid®" works from the inside to lead to plump and firm skin.
*3 Astringent ingredients
*4 Moisturizing
In line with our company philosophy of "continually striving to optimize, not maximize®," we select high-quality, high-tech development partners to align with the value we aim to provide to our customers. Additionally, we engage in joint development initiatives.

For instance, one of our development partners has a history of facilitating the practical application of research outcomes through industry-academia collaboration with a university that is a national leader in research capabilities and knowledge. They are developing new materials with highly specialized personnel and equipment.
One of the products developed through our cooperative system with partners who possess extensive business experience and substantial research and development capabilities is our flagship product, the sugar lipid care supplement "Terminalia First".
Representative product “Terminalia First”

It is important to consider your health and to make adjustments to your diet if necessary. However, I believe that the experience of enjoying a delicious meal is one of life's great pleasures.
Our objective was to develop a product that would allow our customers to enjoy delicious food without any worries, in a simpler, healthier way. With this in mind, we began searching for ingredients that could control sugar and fat.
In developing new materials, while there are many plants in the world whose efficacy is not yet clear, we conducted a comprehensive search for plants that contribute to health, such as sesame and peas. Our research included over 100 plants from Japan and overseas.
The fruit ultimately selected was the Terminalia bellirica, which is indigenous to tropical regions, particularly in South Asia.
This traditional fruit, which features in Indian mythology and ancient texts, is now attracting global attention, not only in India but around the world.
Our team has successfully extracted an extract containing gallic acid from the berries of Terminalia bellirica. We are now embarking on a full-scale development project to further optimize the process.
During the product development process, we conducted a comparative analysis of the effects of several different materials and determined that Terminalia bellirica was the most effective.
Terminalia bellirica is reported to have the effect of suppressing the absorption of sugar from food and the subsequent rise in blood sugar levels after meals.
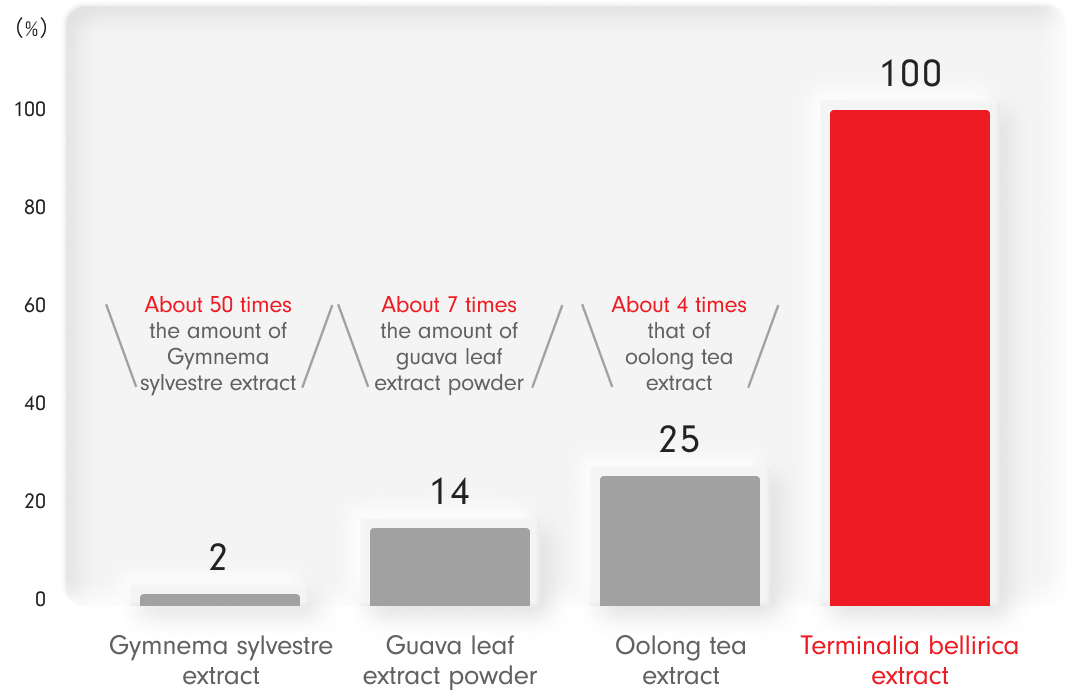
A comparison of carbohydrate absorption inhibition (alpha-glucosidase inhibitory activity potency ratio)
-
The α-glucosidase inhibitory activity of each ingredient was quantified, and an inhibition curve was constructed from the obtained inhibition rate to determine the IC50 of each ingredient.
The ratio of the IC50 of Terminalia bellirica extract to the IC50 of the other ingredients was calculated, and the reciprocal was obtained to determine the ratio of the effects of each ingredient (efficacy ratio) when the efficacy of Terminalia bellirica extract was set at 100%. -
Ryosuke Hama et al. : Safety of functional foods from the perspective of the mode of action of lipase and alpha-glucosidase inhibitory activity of functional ingredients.
Pharmacology and Therapeutics. 48.1261-1265.2020.
Moreover, scientific research has demonstrated that it inhibits the absorption of sugar and fat in the diet.
【Functional superiority】
Please find below the results of the clinical trials conducted on Terminalia bellirica.
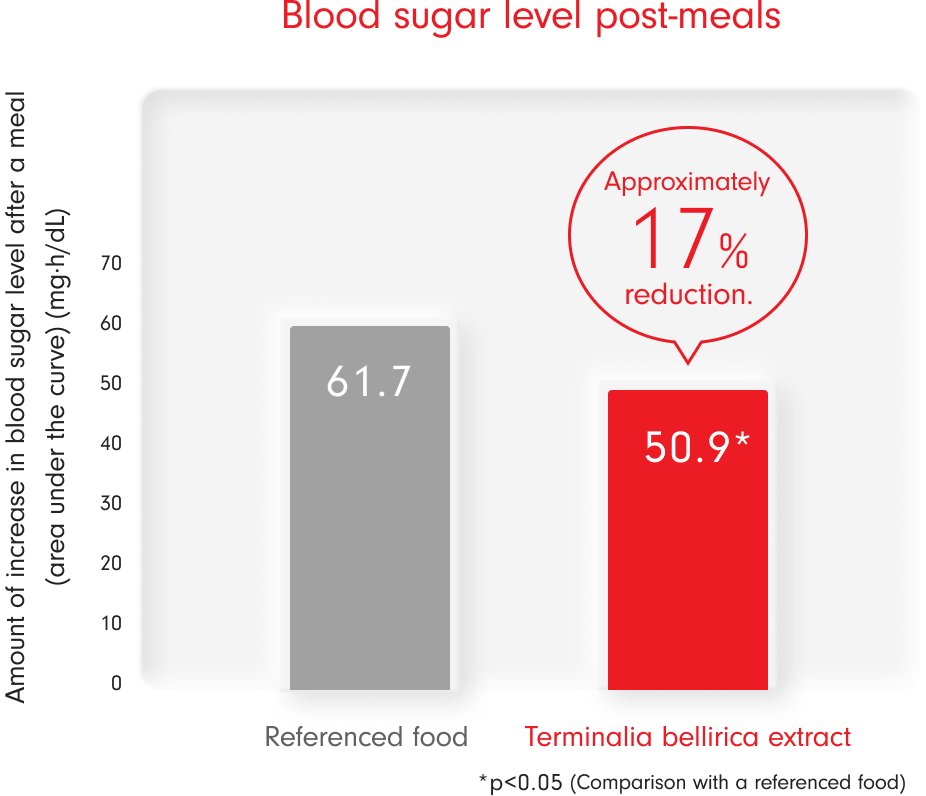
- Method of intake: consumed prior to the test meal (curry and rice).
- Target audience: The target demographic comprises individuals aged between 20 and 65 years old, presenting with fasting blood glucose levels of 100 to 125 mg/dL or 75g OGTT 2-hour values of 140 to 199 mg/dL, and casual blood glucose levels of less than 200 mg/dL. Additionally, the scope encompasses individuals diagnosed with borderline diabetes.
- Number of participants: 46
- The intake of gallic acid derived from Terminalia bellirica was tested at 20.8 mg/day, while the control food had no gallic acid.
- Tomozawa et al. : Pharmacology and Treatment 43(8), 1175-1180, 2015 Reorganized
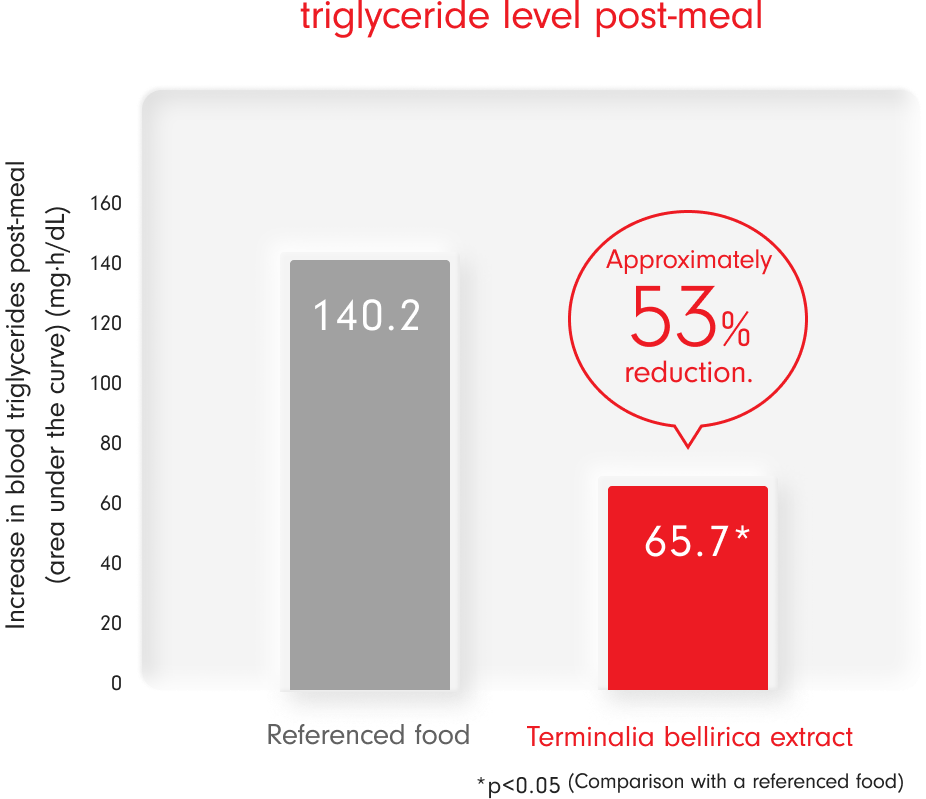
-
Method of intake: Test food or control food, with a diet high in fat (Corn potage with lard and butter, plain bagel) taken before consumption.
The calculation is based on the change in neutral fat levels observed between the time of intake and 6 hours later. - Target: Healthy adults
- Number of participants: 34
- The intake of gallic acid derived from Terminalia bellirica was tested at 20.8 mg/day, while the control food had no gallic acid.
- Kusaba et al. : Pharmacology and Therapeutics, 43(8), 1175-1180, 2015
Following over a decade of product development, the ingredient was approved as a functional food ingredient in 2016.


In addition to its original functions, it has also been shown to suppress the rise in uric acid levels after eating foods high in purines in people with high uric acid levels.
As you can see, even after a product is launched, we continue to conduct a wide range of research on a daily basis, such as clarifying the mechanism of action by verifying the efficacy and safety of functional ingredients.
Thorough quality control based on international standards.
At our company, we are always aware that our customers, as well as ourselves and our families who are our employees, are all "regular users" of our products. Based on the idea of whether we can continue to provide our products to our important families with complete confidence, we aim to maintain and improve quality by focusing on and thoroughly implementing quality control of our products together with our manufacturing partners.
-

First, our products are made from carefully selected, safe ingredients that have been rigorously tested to ensure their purity and efficacy. During the manufacturing process, we use advanced technology and state-of-the-art equipment, and pay close attention to hygiene management.
In addition to inspections at each stage of the process, the final product must pass rigorous quality tests. -

In addition, we adhere to international quality control standards and are subject to regular internal and external third party audits.
-

This allows us to maintain a consistently high level of quality and to deliver our products with confidence in terms of safety and efficacy.
-
We are ISO 22716 (Cosmetics GMP) certified. This is an international standard for quality and safety in the manufacture of cosmetics.
-

VITABRID C POWDER is listed in the International Cosmetic Ingredient Dictionary (ICID).
-

In September 2018, it received a 5-star rating (non-irritating skin certification) from Dermatest, a globally authoritative German dermatological research institute. ※5※6
*5 That doesn't mean it doesn't irritate everyone.
*6 Product:Vitabrid C Hair
Our products are manufactured in a GMP-compliant facility that complies with strict quality control standards.We work to ensure product quality at every stage of the process, from receiving raw ingredients to shipping the finished product.
Below is an example of our quality control system in detail.
Our first priority is to provide high-quality, safe and reliable products, and we are working to provide food safety and security to our customers by strengthening our quality management and improving our quality assurance level in line with the concepts of international standards such as FSSC22000, GMP for dietary supplements, and NSF-GMP.

| Inspection items | Standard value | Test Method |
|---|---|---|
| Foreign matter | No adulteration | The finished tablets are visually inspected to ensure that no foreign material has been introduced. |
| Moisture Value | Below 8% | We use a moisture meter to check the moisture value of the product. |
| General bacterial count | 1.0 x 104 pcs/g or less | The number of bacteria that grow during 48 hours of incubation under aerobic (with oxygen) conditions is counted. |
| coliform bacteria | negative | We test for growth of bacteria in a 24-hour incubation under favorable (oxygenated) conditions. |
- Moisture value: Moisture content affects the stability of the product (e.g. discoloration, deterioration, etc.), so we have established a set of standards to manage it. The norms are determined based on the moisture content of the raw ingredients used.
- Purpose of microbiological testing: The purpose of microbiological testing is to demonstrate that the food is hygienically safe by showing that there are no pathogenic microorganisms present in the food or that the number of microorganisms present is below a certain level.
-
◆Bacterial count: An indicator for assessing the hygienic condition of food
The number of bacteria is set as a guide to the number of bacteria that will cause products to spoil. The initial spoilage of common foods is 1,000,000 cfu/g, but we manage our products to a stricter standard than this. -
◆Coliform bacteria: An indicator of the hygienic condition of food.
It contains many types of naturally occurring bacteria, but we perform testing to quickly identify any risk of containing bacteria that could cause health problems, such as E. coli.
We test for general bacteria and coliform bacteria as part of our routine testing, but if the coliform bacteria test detects bacteria, we use the following equipment to grow the bacteria and identify the species to confirm safety.
-
 Rapid Incubator
Rapid Incubator- Reduces the time required to detect common bacteria, molds and yeasts for fast, accurate detection. Reduced detection time means that anomalies can be detected quickly.
-
 rapid microbial testing device
rapid microbial testing device- ○Possible to identify the species of microorganisms detected, such as E. coli and mold.
- ○Quickly able to determine whether the identified microorganism is a species that can be harmful to health.
For all 43 items, we have confirmed that there are no safety issues after long-term use (3 months).
[Inspected articles]
Systolic Blood Pressure, Diastolic Blood Pressure, Pulse Rate
Hematology test: White blood cell count, Red blood cell count, Hemoglobin, Hematocrit, MCV, MCH, MCHC, Platelet count
Blood Biochemistry:
Total protein, albumin, AST, ALT, LDH, total bilirubin, ALP, γ-GTP, urea nitrogen, creatinine, uric acid, sodium (Na), chloride (Cl), potassium (K), calcium (Ca), phosphorus (P), fasting plasma glucose, HbA1c, insulin, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, phospholipids.
Urinalysis: pH, relative density
Urine Qualitative Testing: Protein, Glucose, Urobilinogen, Bilirubin, Urinary Ketone Bodies, Occult Blood Test
We always make sure to run tests to ensure quality when we receive raw ingredients before they are marketed.
We have thorough measures in place to prevent incorrect entry of ingredients due to mix-ups, etc., and to prevent accidental mixing of ingredients.
[Measures to prevent the incorrect input of ingredients due to mix-ups, etc.]
All ingredients are given a control code and are strictly monitored. During the manufacturing process, the barcodes on the documents and the actual ingredients are scanned, and all must match before work can begin, so there is no risk of mixing up ingredients and using the wrong ones.
[Contamination Control Measures]
We have installed inspection equipment such as magnets and sieves at every stage of the process to prevent the introduction of foreign matter.
[Measures to prevent accidental admixture of ingredients not listed on the ingredient list].
To guarantee that no residual materials from the previous product manufactured on the same production line remain, we conduct a thorough cleaning verification process.
Furthermore, we conduct routine quality control checks to ensure that the cleaning method is effective and that unintended ingredients are not mixed in.
We conduct thorough inspections for contaminants, moisture content, and microbiological concerns at the end of each production run. These inspections ensure that our products meet the highest standards of quality and safety.



















